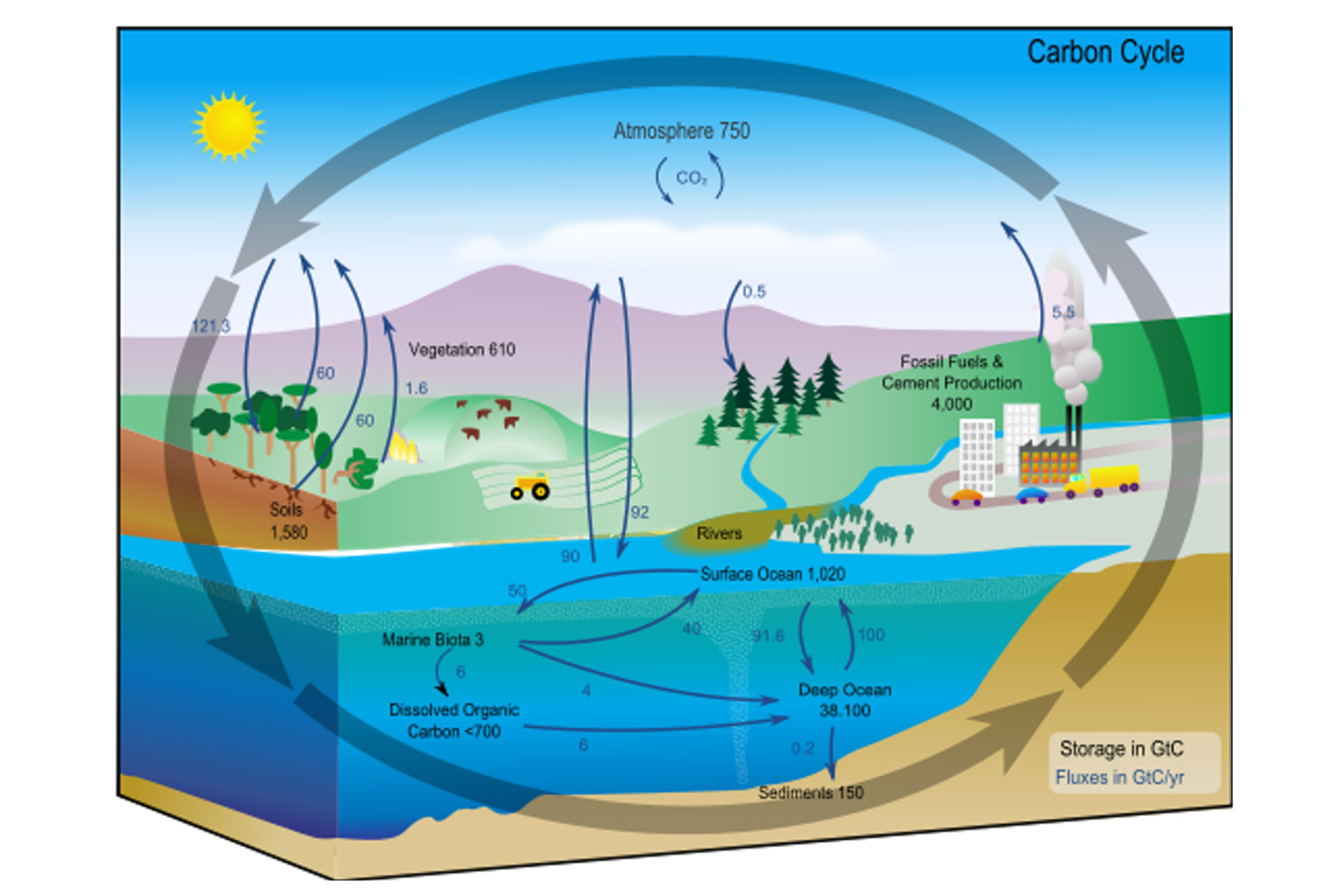
Diagram of the carbon cycle. The black numbers indicate how much carbon is stored in various reservoirs, in billions of tons (“GtC” stands for GigaTons of Carbon and figures are circa 2004). The dark blue numbers indicate how much carbon moves between reservoirs each year. The sediments, as defined in this diagram, do not include the ~70 million GtC of carbonate rock and kerogen.
The Carbon Cycle: the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth.
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. It is one of the most important cycles of the earth and allows for carbon to be recycled and reused throughout the biosphere and all of its organisms. The carbon cycle was initially discovered by Joseph Priestley and Antoine Lavoisier, and popularized by Humphry Davy. It is now usually thought of as including the following major reservoirs of carbon interconnected by pathways of exchange:
The atmosphere
The terrestrial biosphere, which is usually defined to include fresh water systems and non-living organic material, such as soil carbon.
The oceans, including dissolved inorganic carbon and living and non-living marine biota, The sediments including fossil fuels.
The Earth’s interior, carbon from the Earth’s mantle and crust, is released to the atmosphere and hydrosphere by volcanoes and geothermal systems.
The annual movements of carbon, the carbon exchanges between reservoirs, occur because of various chemical, physical, geological, and biological processes. The ocean contains the largest active pool of carbon near the surface of the Earth, but the deep ocean part of this pool does not rapidly exchange with the atmosphere in the absence of an external influence, such as a black smoker or an uncontrolled deep-water oil well leak.
The global carbon budget is the balance of the exchanges (incomes and losses) of carbon between the carbon reservoirs or between one specific loop (e.g., atmosphere ↔ biosphere) of the carbon cycle. An examination of the carbon budget of a pool or reservoir can provide information about whether the pool or reservoir is functioning as a source or sink for carbon dioxide.
2010 Carbon dioxide concentration in the troposphere
Carbon exists in the Earth’s atmosphere primarily as the gas carbon dioxide (CO2). Although it is a small percentage of the atmosphere (approximately 0.04% on a molar basis), it plays a vital role in supporting life. Other gases containing carbon in the atmosphere are methane and chlorofluorocarbons (the latter is entirely anthropogenic). Trees and other green plants such as grass convert carbon dioxide into carbohydrates during photosynthesis, releasing oxygen in the process. This process is most prolific in relatively new forests where tree growth is still rapid. The effect is strongest in deciduous forests during spring leafing out. This is visible as an annual signal in the Keeling curve of measured CO2 concentration. Northern hemisphere spring predominates, as there is far more land in temperate latitudes in that hemisphere than in the southern. – Forests store 86% of the planet’s terrestrial above-ground carbon and 73% of the planet’s soil carbon.
At the surface of the oceans towards the poles, seawater becomes cooler and more carbonic acid is formed as CO2 becomes more soluble. This is coupled to the ocean’s thermohaline circulation, which transports dense surface water into the ocean’s interior (see the entry on the solubility pump). In upper ocean areas of high biological productivity, organisms convert reduced carbon to tissues, or carbonates to hard body parts such as shells and tests. These are, respectively, oxidized (soft-tissue pump) and redissolved (carbonate pump) at lower average levels of the ocean than those at which they formed, resulting in a downward flow of carbon (see entry on the biological pump).
The weathering of silicate rock (see carbonate-silicate cycle). Carbonic acid reacts with weathered rock to produce bicarbonate ions. The bicarbonate ions produced are carried to the ocean, where they are used to make marine carbonates. Unlike dissolved CO2 in equilibrium or tissues which decay weathering does not move the carbon into a reservoir from which it can readily return to the atmosphere.
In 1958, atmospheric carbon dioxide at Mauna Loa was about 320 parts per million (ppm), and in 2011 it is about 391ppm.[3] – Future CO2 emission can be calculated by the kaya identity.
Carbon is released into the atmosphere in several ways:
Through the respiration performed by plants and animals. This is an exothermic reaction and it involves the breaking down of glucose (or other organic molecules) into carbon dioxide and water.
Through the decay of animal and plant matter. Fungi and bacteria break down the carbon compounds in dead animals and plants and convert the carbon to carbon dioxide if oxygen is present, or methane if not.
Through combustion of organic material, which oxidizes the carbon it contains, producing carbon dioxide (and other things, like water vapor). Burning fossil fuels such as coal, petroleum products, and natural gas releases carbon that has been stored in the geosphere for millions of years. Burning agro-fuels also releases carbon dioxide, which has been stored for only a few years or less.
Production of cement. Carbon dioxide is released when limestone (calcium carbonate) is heated to produce lime (calcium oxide), a component of cement.
At the surface of the oceans where the water becomes warmer, dissolved carbon dioxide is released back into the atmosphere.
Volcanic eruptions and metamorphism release gases into the atmosphere. Volcanic gases are primarily water vapor, carbon dioxide and sulfur dioxide. The carbon dioxide released is roughly equal to the amount removed by silicate weathering;[citation needed] so the two processes, which are the chemical reverse of each other, sum to roughly zero, and do not affect the level of atmospheric carbon dioxide on time scales of less than about 100,000 years.
In the biosphere
Carbon is an essential part of life on Earth. About half the dry weight of most living organisms is carbon. It plays an important role in the structure, biochemistry, and nutrition of all living cells. Living biomass holds about 575 gigatons of carbon, most of which is wood. Soils hold approximately 1,500 gigatons,[4] mostly in the form of organic carbon, with perhaps a third of that inorganic forms of carbon such as calcium carbonate.[5]
Autotrophs are organisms that produce their own organic compounds using carbon dioxide from the air or water in which they live. To do this they require an external source of energy. Almost all autotrophs use solar radiation to provide this, and their production process is called photosynthesis. A small number of autotrophs exploit chemical energy sources in a process called chemosynthesis. The most important autotrophs for the carbon cycle are trees in forests on land and phytoplankton in the Earth’s oceans. Photosynthesis follows the reaction 6CO2 + 6H2O → C6H12O6 + 6O2
Carbon is transferred within the biosphere as heterotrophs feed on other organisms or their parts (e.g., fruits). This includes the uptake of dead organic material (detritus) by fungi and bacteria for fermentation or decay.
Most carbon leaves the biosphere through respiration. When oxygen is present, aerobic respiration occurs, which releases carbon dioxide into the surrounding air or water, following the reaction C6H12O6 + 6O2 → 6CO2 + 6H2O. Otherwise, anaerobic respiration occurs and releases methane into the surrounding environment, which eventually makes its way into the atmosphere or hydrosphere (e.g., as marsh gas or flatulence).
Burning of biomass (e.g. forest fires, wood used for heating, anything else organic) can also transfer substantial amounts of carbon to the atmosphere – Carbon may also be circulated within the biosphere when dead organic matter (such as peat) becomes incorporated in the geosphere. Animal shells of calcium carbonate, in particular, may eventually become limestone through the process of sedimentation.
Much remains to be learned about the cycling of carbon in the deep ocean. For example, a recent discovery is that larvacean mucus houses (commonly known as “sinkers”) are created in such large numbers that they can deliver as much carbon to the deep ocean as has been previously detected by sediment traps.[6] Because of their size and composition, these houses are rarely collected in such traps, so most biogeochemical analyses have erroneously ignored them.
Carbon storage in the biosphere is influenced by a number of processes on different time-scales: while net primary productivity follows a diurnal and seasonal cycle, carbon can be stored up to several hundreds of years in trees and
up to thousands of years in soils. Changes in those long term carbon pools (e.g. through de- or afforestation or through temperature-related changes in soil respiration) may thus affect global climate change.
In the hydrosphere (incomplete)
“Present day” (1990s) sea surface dissolved inorganic carbon concentration (from the GLODAP climatology)
The oceans contain around 36,000 gigatonnes of carbon, mostly in the form of bicarbonate ion (over 90%, with most of the remainder being carbonate). Extreme storms such as hurricanes and typhoons bury a lot of carbon, because they wash away so much sediment. For instance, a research team reported in the July 2008 issue of the journal Geology that a single typhoon in Taiwan buries as much carbon in the ocean—in the form of sediment—as all the other rains in that country all year long combined. Inorganic carbon, that is carbon compounds with no carbon-carbon or carbon-hydrogen bonds, is important in its reactions within water. This carbon exchange becomes important in controlling pH in the ocean and can also vary as a source or sink for carbon. Carbon is readily exchanged between the atmosphere and ocean. In regions of oceanic upwelling, carbon is released to the atmosphere. Conversely, regions of downwelling transfer carbon (CO2) from the atmosphere to the ocean. When CO2 enters the ocean, it participates in a series of reactions which are locally in equilibrium:
Solution: CO2(atmospheric) ⇌ CO2(dissolved)
Conversion to carbonic acid: CO2(dissolved) + H2O ⇌ H2CO3 First ionization: H2CO3 ⇌ H+ + HCO3− (bicarbonate ion) Second ionization: HCO3− ⇌ H+ + CO32− (carbonate ion)
This set of reactions, which of each has its own equilibrium coefficient, determines the form that inorganic carbon takes in the oceans. The coefficients, which have been determined empirically for ocean water, are themselves functions of temperature, pressure, and the presence of other ions (especially borate). In the ocean the equilibria strongly favor bicarbonate. Since this ion is three steps removed from atmospheric CO2, the level of inorganic carbon storage in the ocean does not have a proportion of unity to the atmospheric partial pressure of CO2. The factor for the ocean is about ten: that is, for a 10% increase in atmospheric CO2, oceanic storage (in equilibrium) increases by about 1%, with the exact factor dependent on local conditions. This buffer factor is often called the “Revelle Factor”, after Roger Revelle.
In the oceans, dissolved carbonate can combine with dissolved calcium to precipitate solid calcium carbonate, CaCO3, mostly as the shells of microscopic organisms. When these organisms die, their shells sink and accumulate on the ocean floor. Over time these carbonate sediments form limestone which is the largest reservoir of carbon in the carbon cycle. The dissolved calcium in the oceans comes from the chemical weathering of calcium- silicate rocks, during which carbonic and other acids in groundwater react with calcium-bearing minerals liberating calcium ions to solution and leaving behind a residue of newly formed aluminium-rich clay minerals and insoluble minerals such as quartz.
The flux or absorption of carbon dioxide into the world’s oceans is influenced by the presence of widespread viruses within ocean water that infect many species of bacteria. The resulting bacterial deaths spawn a sequence of events that lead to greatly enlarged respiration of carbon dioxide, enhancing the role of the oceans as a carbon sink.
There is a big difference in waters i.e. pure H2O, drinking water (river water), and salt water
The missing processes in the carbon cycle that create drinking water (river water)
What is pristine drinking water? What are the chemical processes involved in nature that bring us pristine drinking water. And how has acid rain disrupted the hydrological and carbon cycles that are so detrimental in bringing us pristine drinking water that contains optimal and relative electrolyte bicarbonates salts?
H2O (water) and CO2 (carbon dioxide) make H2CO3 (carbonic acid) The chemical equation is: H2O + CO2 => H2CO3
Carbonic acid is a naturally occurring acid (a positive ion). Carbonic acid is stable in river water a four degrees Celsius. This naturally occurring acid erodes the earth. For example 98.5 % of the earths crust is made up of eight elements seven of them are minerals, oxygen, silica, alumina, iron, calcium, sodium, potassium and magnesium.
Obviously, carbonic acid water (a natural solvent) releases minerals optimally and relatively according to the abundance of the mineral in the earths crust and it specific density.
For example the carbonic acid water releases and tiny bit of silica and alumina because that are very hard. It released some iron (iron is the third most prevalent mineral in the earths crust and the number one element on the planet). Then the carbonic acid water releases calcium, sodium, potassium, and magnesium, the initial elements releases are in the form of oxides. As the carbonic acid water is flowing down the river it encounters vortexes and as the water and oxides are imploding it knocks of the oxygen off the calcium, sodium, potassium, and magnesium oxides and it becomes a carbonate (a carbonate is an oxide minus the oxygen). As the carbonates are rushing down the river (carbonic acid water) the carbonates are reduced in molecular size to hydroxides. This is as small as they are going to get.
As we mentioned earlier, carbonic acid (H2CO3) is stable in river water a four degrees Celsius. When I warms up the carbonic acid will slightly and reversible drops a hydrogen ion (it dissociate a hydrogen ion) and it becomes a bicarbonate ion HCO3 (a negative ion).
The chemical equation is:
H2O (water) + CO2 (carbon dioxide) => H2CO3 (carbonic acid) H2CO3 (carbonic acid) – H (hydrogen) => HCO3 (a bicarbonate ion)
Bicarbonate ions (negative ions) are like magnets and they have a strong affinity for the positively charged calcium, sodium, potassium, and magnesium hydroxides. This is the process that creates bicarbonate electrolyte salts. These bicarbonate salts have nothing to do with building bones, muscles, organs, or blood.
They are alkaline electrolyte salts and they facilitate voltage gated ion channels in the body. This is a process that naturally occurred and it resulted in slightly alkaline pristine water that contained electrolyte salts. It is called water! Acid free water (the carbonic acid dissociate the hydrogen and it became a bicarbonate ion) that contained four electrolyte salts.
Obviously, bicarbonate salts and water (acid free) were two of the most natural compounds on earth.
In 1964, only 1 person in 214 contracted Cancer. Today it is 1 in 3 females and 1 in 2 males. The determining factor between health and disease is pH. It is not uncommon for the average American to test between 4 pH and 5 pH.
- Oxygen levels in the body are directly related to pH.
- Increasing pH from 4 pH to 5 pH increases oxygen to the cells by ten fold.
- Increasing pH from 4 to 6 increases oxygen by 100 times and raising pH from 4 pH to 7 pH increases oxygen levels by 1,000 times.
- Cancer cells have an extreme acid ph and are oxygen depleted while healthy cells have a slightly alkaline pH with a high oxygen content.
Research shows that unless the body’s pH level is slightly alkaline, the body cannot heal itself. So, no matter what type of modality you use to improve your health problem, the modality won’t be effective until the pH level comes up.
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole (generalized hypoxia) or a region of the body (tissue hypoxia) is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise. A mismatch between oxygen supply and its demand at the cellular level may result in a hypoxic condition. Hypoxia in which there is complete deprivation of oxygen supply is referred to as anoxia.
Hypoxia differs from hypoxemia in that, in the latter, the oxygen concentration within the arterial blood is abnormally low. It is possible to experience hypoxia and have a low oxygen content (e.g., due to anemia) but maintain high oxygen partial pressure (pO2). Incorrect use of these terms can lead to confusion, especially as hypoxemia is among the causes of hypoxia (in hypoxemic hypoxia).
Generalized hypoxia occurs in healthy people when they ascend to high altitude (or chronic acidosis or elevated nitrogen levels), where it causes altitude sickness leading to potentially fatal complications: high altitude pulmonary edema (HAPE) and high altitude cerebral edema (HACE).
Hypoxia is also a serious consequence of preterm birth in the neonate. The main cause for this is that the lungs of the human foetus are among the last organs to develop during pregnancy. To assist the lungs to distribute oxygenated blood throughout the body, infants at risk of hypoxia are often placed inside an incubator capable of providing continuous positive airway pressure (also known as a humidicrib).
Who is at risk from high nitrates in drinking water?
The Environmental Protection Agency (EPA) has set the Maximum Contaminant Level (MCL) of nitrate as nitrogen (NO3-N) at 10 mg/L (or 10 parts per million) for the safety of drinking water. Nitrate levels at or above this level have been known to cause a potentially fatal blood disorder in infants under six months of age called methemoglobinemia or “blue-baby” syndrome; in which there is a reduction in the oxygen-carrying capacity of blood. The symptoms of blue-baby syndrome can be subtle and often confused with other illnesses. An infant with mild to moderate blue-baby syndrome may have diarrhea, vomiting, and/or be lethargic. In more serious cases, infants will start to show obvious symptoms of cyanosis: the skin, lips or nailbeds may develop a slate-gray or bluish color and the infant could have trouble breathing. A sample of the infant’s blood can easily confirm a diagnosis of blue-baby syndrome. It is difficult to determine the true incidence of blue-baby syndrome in Washington State because it is not a reportable disease.
Blue baby syndrome is an environmentally-caused children’s health issue. Blue baby syndrome, or methemoglobinemia, is an illness that arises when an infant’s blood is unable to carry enough oxygen to body cells and tissue. It is caused by a rise in the level of methemoglobin in the blood. Methemoglobin is a non-oxygen- carrying enzyme that is continually produced in the body. It is converted to hemoglobin, the oxygen-carrying enzyme in the blood, by a red blood cell enzyme called methemoglobin reductase. Because infants under that age of six months have little methemoglobin reductase in their systems, an excess of methemoglobin, or methemoglobinemia, can be fatal if left untreated.
Excessive nitrates in drinking water can adversely affect children’s health, sometimes causing blue baby syndrome. When ingested, these nitrates are converted to nitrite in the digestive system; these nitrites react with the hemoglobin in the blood, forming high amounts of methemoglobin. Since methemoglobin cannot carry oxygen, if enough too much of the enzyme is in the blood, the infant’s tissue and organs may be deprived of oxygen. This will cause him or her to develop a bluish coloring and possibly result in long-term digestive and respiratory problems.
Because vegetables, including green beans, carrots, squash, spinach and beets, can have nitrate levels as high or higher than that of well water, infants should not eat these foods until after age 3 months.
There are high nitrates in NPK (nitrogen, phosphate, and potassium fertilizer), which leach into our water supply and are incorporated in the vegetables we eat.
Chronic malnutrition, sugar, processed food, plastic fats, calcified pineal gland; acid pH, nitrates and decreased oxygen levels equal hypoxia.